The BEST data.
Do what is right and not what is “always been done”
How it works - step by step
Define the proper physiologically relevant conditions for your clinical target or other application (such as 3D bioprinting)
Subject the specimen (biomaterial, tissue, injectable mateial, construct) to coherent stimuli, inducing respective fluid and nutrients transport closer to what is expected in the hostile environment
Assess changes in properties of the specimen in time, phase and stimulus domains, leading to the fundamental and invariant constitutive equation for given material, generating model-free data (no assumptions or artificial parameters)
Post-process experimental readouts with idempotent analysis (patented and patents pending), incorporating specimen history.
Extract invariant values, suitable for material behavior prediction, showing also the position and the direction of development of your material and provide solid data for its optimization.
Already tested? No problem.
When you already have data from previous experiments and if these conditions are relevant, BEST can extract the same or similar values from your data.
Please contact us for details as missing data and improper readouts may not be feasible for analysis
STILL UNSURE?
Please ask! Will be happy to discuss details. NDA is possible if data are too sensitive.
Key BEST features:
Shorten time and costs of materials development and optimization
Decrease/eliminate needs for extra animal testing
Characterize at coherent conditions, closer to physiological reality.
Eliminate assumptions on material linearity, homogeneity, material model
Enable high-output screening (instead of high-throughput screening)
Incorporate specimen history dependence and making prediction possible
Carry out a non-destructive evaluation in most cases - you may reprocess the specimen
Avoid usage of complex numbers or transforms - no troubles from harmonics or series expansion
Re-process retrospective data without need for new experiments
Incorporate drug transport signature (DTS) features when your material is a medicinal product or having CDR feature
BEST measures properties for the purpose - the same material may have different properties vs. conditions like media, temperature, etc., so it is important the values are exactly what you need for that medical application your material has its intended purpose.
Some BEST cases
-
New treatment of ceramic dental abutments for better aesthetics and improved mucosal adhesion, minimizing bacterial contamination risk.
OUTCOME: new abutments for clinical trials.
More: see BEST notes in recent publications (J. Periodontology and in MDPI Biomaterials) -
discovering new auto-mechano-induction (US Patent) effect in different cells development, proving ability to trigger changes in cells reaction to anticancer drugs.
OUTCOME: new special models for stem and cancer cell culturing under development. Recently tested for different viruses too (see publication in Materials) -
development of new scaffolds with proper biomechanic-fluidic properties polarization for better hyaline cartilage regeneration.
OUTCOME: new optimized scaffolds are being deployed in clinical practice.
See BEST notes for joints in the publication (Frontiers Bioeng. Biotech.) for details -
Revealed changes in new hyaluronic hydrogels with bioactive fillers affecting viscosity and stiffness, and how to manage injectability (US patent) and patient comfort.
OUTCOME: new hydrogels compositions in preparation. Bioprinting applications under development.
BEST Applications and Values
With the proper coherent testing and data analysis BEST generates in vitro evidence which
lowers the costs and time to get the correct data
provides outcomes as true real properties with better biofidelity and higher clinical/physiological relevance
enables model-free prediction of a specimen behavior and its unbiased comparison with the controls
with the high-output screening of lead solutions provides data for RAQA and regulatory compliance
raises ethical value of your biomedical product.
BEST data are needed for:
regulative compliance validation as with MDR 2017/745 (Annex I),
model-free prediction of behavior for specific clinical application,
lead development/lead optimization of the biomedical solutions,
new features discovery,
reduction, refinement and replacement ("3R") of animal testing (2010/63/EC).
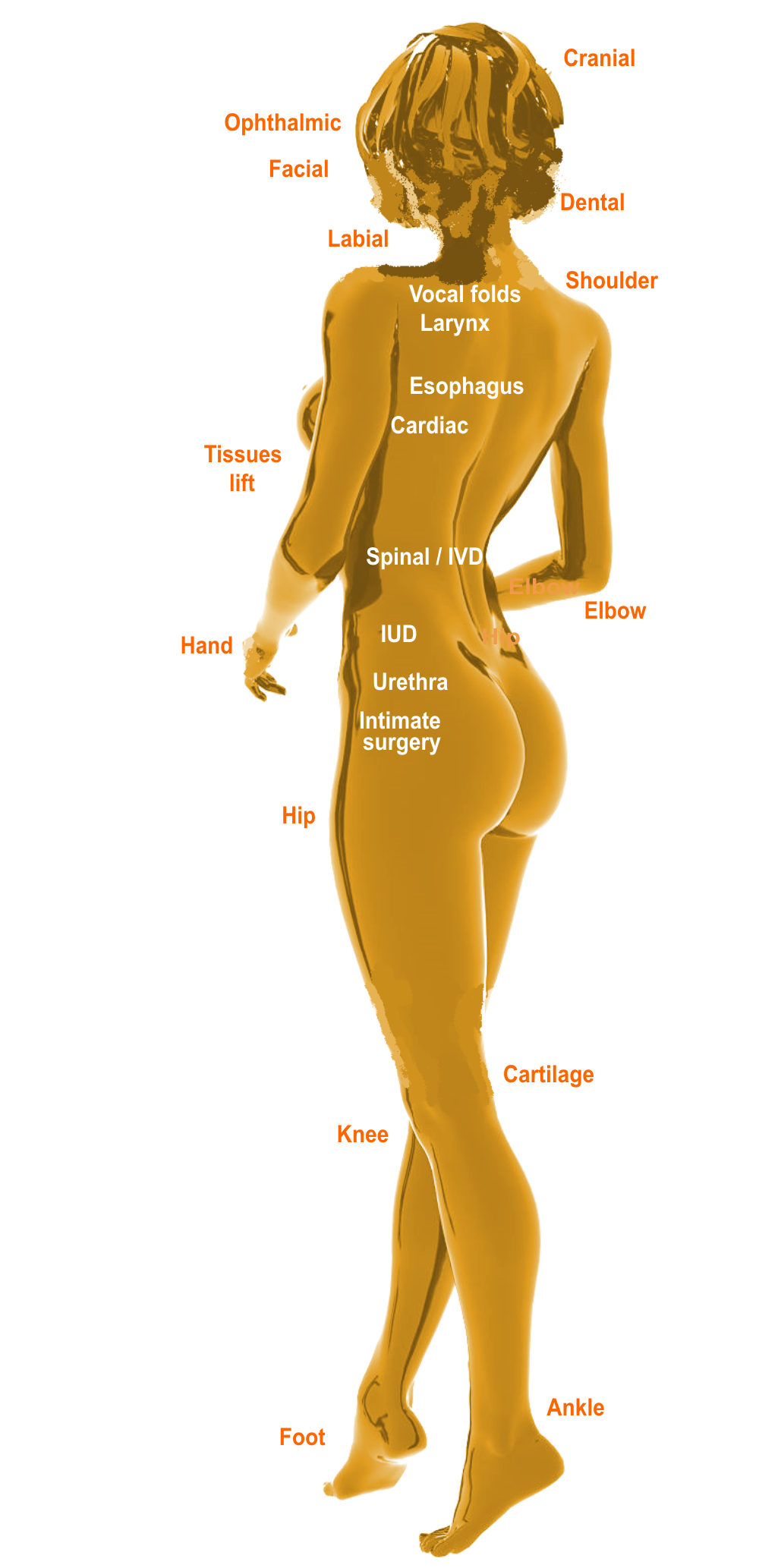
BEST applications span many medical targets, with a great variety of the functionality, optimization and risk assessment actions:
Tissues
Polymers
Hydrogels
Injectables
Decellurized
Bioceramics
Biometals
Phantoms
Composites
Hybrids
Drugs and CDR
. . . . .
Coherent testing
Idempotent analysis
Model-free data
Predictive outcomes
. . . . .
Clinical targets with BEST
3D bioprinting
ATMP solutions
Anti-bacterial
Bio-inks
Cancer
Cardiac
Cosmetic
Dental
Gynaecologic
Immunologic
Implants
Injectables
Joints repair
Neurologic
Ophthalmic
Orthobiology
Orthopaedic
Pharmaceutics
Plastic surgery
Pulmonologic
Scaffolds
Soft tissues
Spinal / IVD
Surgical
Urologic
Veterinary
Please inquire for customized solutions !